Welcome to Cancer Biophysics Lab
The fundamental cause of cancer is damaged or faulty genes – the instructions that tell cells what to do. Genes are encoded within DNA, so anything that damages DNA can increase the risk of cancer. However, a number of genes in the same cell need to be damaged before it can become cancer.
Photo Gallery




About Me
Selected Publications
Research Interests
I am a physical chemist and the Director of General Chemistry at the Chemistry Department of UIC with research interests in cancer biophysics. I hold a Ph.D. in Biophysical Chemistry and a Master's of Science in Computer Science. I teach General, Analytical and Physical Chemistry at the University of Illinois Chicago and I have develo
I am a physical chemist and the Director of General Chemistry at the Chemistry Department of UIC with research interests in cancer biophysics. I hold a Ph.D. in Biophysical Chemistry and a Master's of Science in Computer Science. I teach General, Analytical and Physical Chemistry at the University of Illinois Chicago and I have developed an introductory course in Bioinformatics
------------------------
I have taught
CHEM 116/118 Honors General Chemistry
CHEM 124 (114) General Chemistry II
CHEM 222 Analytical Chemistry
CHEM 342/346 Phys. Chem. I and II
CHEM 340/344 Phys. Chem.for Biochemists I and II
CHEM 343 Phys. Chem. Lab
CHEM 402 Chemical Information Systems
----------------
I have co-authored a textbook of physical chemistry. The original book was published 113 years ago.
________________
Affiliations
American Chemical Society
American Physical Society
Biophysical Society

Research Interests
Selected Publications
Research Interests
The overall objective of my research is to understand the mechanisms and consequences of damage in DNA, either in simple mononucleotides or in oligomers. We employ ab initio quantum mechanical calculations to investigate the DNA damage induced by ionizing UV radiation and alkylating electrophiles.
Nucleotide ionization energies
The overall objective of my research is to understand the mechanisms and consequences of damage in DNA, either in simple mononucleotides or in oligomers. We employ ab initio quantum mechanical calculations to investigate the DNA damage induced by ionizing UV radiation and alkylating electrophiles.
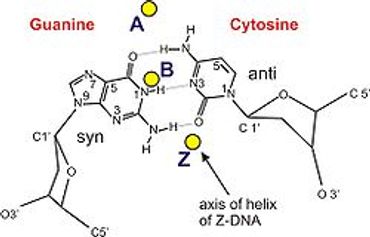
Nucleotide ionization energies provide a quantitative measurement of the electron-donating properties of DNA and they are the key initial steps than can lead to direct DNA damage and mutation. Moreover, many cancer-causing agents are electrophiles that act by attacking DNA and alkylating it.
The susceptibility of nucleotides to electrophilic attack plays also a ubiquitous role in mechanisms of chemical carcinogenesis and cancer chemotherapy.
It is established that in gliomas and colorectal carcinomas, aberrant DNA methylation was detected in 40% of the tumors, whereas in non-small cell lung carcinomas, lymphomas, and head and neck carcinomas, this alteration was found in 25% of the tumors.

Selected Publications
Selected Publications
Selected Publications
1) M. V. Yermolina, G. A. Papadantonakis, " A computational investigation of cytosine and 5-methyl cytosine reactivity by means of ionization potentials and one specific methylation pathway"
Chemical Physics Letters, 2020, 752, 137544
2) M. V. Yermolina, G. A.Papadantonakis,
" Electron and radical cation of sulfur-substituted thym
1) M. V. Yermolina, G. A. Papadantonakis, " A computational investigation of cytosine and 5-methyl cytosine reactivity by means of ionization potentials and one specific methylation pathway"
Chemical Physics Letters, 2020, 752, 137544
2) M. V. Yermolina, G. A.Papadantonakis,
" Electron and radical cation of sulfur-substituted thymine derivatives produced near photoionization threshold can alter and distort double-helix DNA structure"
Chemical Physics Letters, 2019, 737, 136831
3) A. Rojas, S. Cabal, G. A. Papadantonakis,
" A theoretical investigation of sugar and phosphate contributions to the activation barriers of guanine methylation by carcinogenic methane diazonium ion"
Chemical Physics Letters, 2019, 714, 24-2
4) D. R. Eichler, G. A. Papadantonakis, “Methylation of DNA Bases by Dimethyl Sulfate,”
Chemical Physics Letters, 689, 8-14, (2017)
5) D. R. Eichler, H. A. Hamann, K. A. Harte, G. A. Papadantonakis, “Hydration effects on the photoionization energy of 2’-deoxyguanosine 5’-phosphate and activation barriers for guanine methylation by carcinogenic methane diazonium ions,”
Chemical Physics Letters, 680, 83-89, (2017)
6) A. F Philip, R. A. Nome, G. A. Papadantonakis, N. F. Scherer Wouter D. Hoff, “Spectral Tuning in Photoactive Yellow Protein by Modulation of the Shape of the Excited State Energy Surface,“
Proceedings of National Academy of Science USA,, 107, 5821-5826, (2010), DOI: 10.1073/pnas.0903092107
7) A.F. Philip, K.T. Eisenman, G. A. Papadantonakis, W. D. Hoff, “Functional Tuning of Photoactive Yellow Protein by Active Site 46,”
Biochemistry, 47, 13800-13810, (2008) DOI: 10.1021/bi801730y
8) G. A. Papadantonakis, R. S. Tranter, K. Brezinsky, Y. Yang, R. B. van Breemen, P. R. LeBreton, “Low-Energy, Low-Yield Photoionization and Production of 8’-Oxo-2’-deoxyguanosine and Guanine from 2’-Deoxyguanosine,”
Journal of Physical Chemistry B, 106, 7704-7712 (2002)., DOI: 10.1021/jp0206491
9) G. A. Papadantonakis, K. L. Stevenson, P. R. LeBreton, “Evidence of Temperature Dependent Activation Barriers for Near-Threshold Aqueous Photoionization of 2’-Deoxyguanosine and Tryptophan,”
Chemical Physics Letters 346, 97-102, (2001). DOI: 10.1016/S0009-2614(01)00868-5
10) K. L. Stevenson, G. A. Papadantonakis, P. R. LeBreton, “Nanosecond UV Laser Photoionization of Aqueous Tryptophan: Temperature Dependence of Quantum Yield, Mechanism, and Kinetics of Hydrated Electron Decay,”
Journal of Photochemistry and Photobiology A: Chemistry 133(3), 159-167, (2000), DOI: 10.1016/S1010-6030(00)00235-5
11) H. Fernando, G. A. Papadantonakis, N.S. Kim, Pierre R. LeBreton,” Conduction-Band-Edge Ionization Thresholds for DNA Components in Aqueous Solutions,” Proceedings of National Academy of Science USA, 95, 5550-5555, (1998). DOI: 10.1073/pnas.95.10.5550
Teaching Philosophy

Grab interest
I am quite passionate about teaching because I would like to make a difference in the lives of my students. I developed three broad teaching goals that I discuss below. The first is to instill in students a sense of enthusiasm for the study of chemistry. The second is to help students build the basic critical thinking skills that they need both in college and in their post-college careers. The third is to foster in students the understanding that chemistry is not merely a series of topics to memorize but a constantly evolving science that addresses the challenges of every aspect of human life.

Generate excitement
I believe that my role in teaching chemistry courses is to serve both as a “communicator” by delivering lectures, giving examinations and assigning grades and, as a ”motivator” by motivating students to do homework problems and spend time in the laboratory learning basic experimental techniques that will help them to perform scientific research.

Epitome
In today’s world, computer technology is an essential component of chemistry and students should use it where it can enhance their understanding beyond traditional teaching methods.
Contact
University of Illinois at Chicago
Department of Chemistry, 845 W. Taylor St., Room 4500 SES, Chicago, IL. 60607